The Food and Drug Administration is now considering Merck's COVID-19 antiviral pill. However, the health agency said they would still check the drug before recommending it for usage.

A researcher works on a vaccin against the new coronavirus COVID-19 at the Copenhagen's University research lab in Copenhagen, Denmark, on March 23, 2020. - At Copenhagen university, a team of about 10 researchers is working around the clock to develop a vaccine against Covid-19 that could apply for clinical trial before within nine months. The vaccine will be based on two components.
But, if FDA ever approved the new antiviral medicine, it would make a huge difference during the ongoing global pandemic, especially since health experts discovered a new COVID-19 variant that could be more infectious compared to the Delta type.
Right now, various health firms are now making efforts to prevent the further spread of the new Omicron variant. Recently, TechTimes reported that there are some ways to protect yourself against the new COVID-19 Omicron.
On the other hand, Moderna's new vaccines would specifically be developed for the new virus.
Merck's COVID-19 Antiviral Pill Considered by FDA?
According to NPR's latest report, if FDA decided to approve Merck's COVID-19 antiviral medicine, it would be the first take-at-home pills against the novel coronavirus.
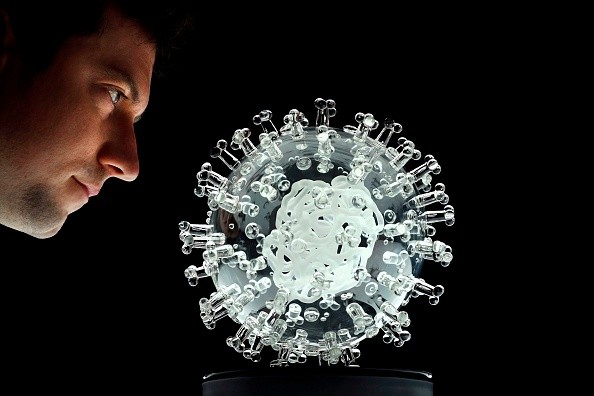
A glass sculpture entitled "coronavirus COVID-19" created by British artist Luke Jerram is seen at his studio in Bristol, southwest of England on March 17, 2020. - Jerram has created a coronavirus COVID-19 glass sculpture in tribute to the huge global scientific and medical effort to combat the pandemic. Made in glass, at 23cm in diameter, it is 1 million times larger than the actual virus.
Also Read: Pfizer, Moderna to 'Update' Vaccines to Work Against New Extremely Infectious Variants, WHO Warns
"With omicron [variant] breathing down our necks, we need drugs, we need really effective antivirals, and we need more of them," said NIAID Director of the Division of AIDS, Carl Dieffenbach, who is also the antiviral development's leader.
Aside from Merck's product, there are also other antiviral pills now being developed. These include the new Paxlovid medicine of Pfizer, which manufactures one of the most efficient vaccines.
Pfizer's antiviral drug is expected to reduce COVID-19 deaths, as reported by USA Today. You can view this link to see more details.
Molnupiravir's Efficiency
Merck's COVID-19 oral pill called Molnupiravir is claimed to reduce COVID-19 deaths and hospitalization, which is quite similar to Pfizer's version.
The developers explained that if patients correctly take the pills within five days after their symptoms appear, it Molnupiravir would have an efficiency of 30%.
However, the actual performance of the new medicine would depend on whether the Food and Drug Administration actually approves it.
For more news updates about COVID-19 and other health topics, always keep your tabs open here at TechTimes.
Related Article: Could a Gum be a COVID-19 Stopper? Here's Everything You Need to Know About this Potential Solution
TechTimes own this article
Written by: Griffin Davis









