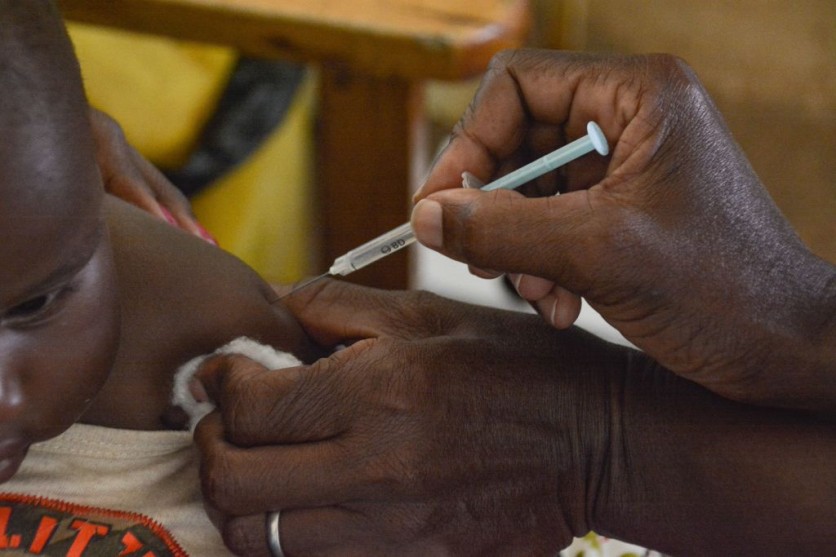
The World Health Organization (WHO) has authorized the development of a second malaria vaccine, marking a breakthrough that may provide developing nations with a more accessible, affordable, and effective option to battle this parasitic illness.
In announcing this significant progress, WHO Director-General Tedros Adhanom Ghebreyesus emphasized the importance of the decision, particularly for young people at risk for malaria. A malaria researcher, Tedros expressed his excitement, saying, "I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two," as quoted by AP News.
The Serum Institute of India and Oxford University worked together to create this second malaria vaccine, which is administered in a three-dose course. According to research, it is more than 75% effective and, when enhanced, protects for at least an extra year. It is predicted that each dosage will cost between $2 and $4.
This second malaria vaccine may be made available in a few nations as early as next year, pending financial arrangements. Regulating bodies in Ghana and Burkina Faso had previously approved this malaria vaccination earlier in the year.
Vaccine Will Not End Malaria, Expert Warns
Meawhile, John Johnson of Doctors Without Borders stressed that the new malaria vaccine should not replace bed nets and pesticides as malaria prevention methods. "This is not the vaccine that is going to stop malaria," he remarked.
According to Reuters, the Serum Institute of India mass produces the R21/Matrix-M vaccine, which includes the Matrix M adjuvant from Novavax. CEO of the Serum Institute of India, Adar Poonawalla, said that the company has generated over 20 million doses in advance of the WHO's guidance.
This new malaria vaccine will compete with GSK plc's (GSK.L) RTS, S injection, and Mosquirix, which obtained WHO endorsement in 2021. In independent trials, both vaccinations showed equal effectiveness, but there has been no direct comparison to establish which is superior. Because of this, WHO has given each countries the freedom to choose its own vaccine, taking into consideration cost and availability.
In a statement, GSK recognized the need for a second malaria vaccine and emphasized that the RTS has "set a strong benchmark" as the first-ever malaria vaccine to protect against a human parasite.
Malaria Vaccines To Save Hundreds of Thousands
The RTS-S vaccine has been given to over 1.7 million children in Ghana, Kenya, and Malawi, with plans to extend to nine more malaria-endemic countries next year.
The WHO regional director for Africa, Dr. Matshidiso Moeti, stressed the potential of this second vaccination to close the huge gap between supply and demand. "Delivered to scale and rolled out widely, the two vaccines can help bolster malaria prevention, control efforts and save hundreds of thousands of young lives," Moeti said, as quoted by the BBC.
Preliminary data shows that the R21 vaccine is 75% effective in seasonal malaria regions, matching the first vaccine, RTS. However, in areas where malaria is transmitted year-round, vaccination efficacy generally tends to be lower.
Related Article : COVID-19 mRNA Vaccine Pioneers, Katalin Kariko and Drew Weissman, Clinch Nobel Medicine Prize

ⓒ 2026 TECHTIMES.com All rights reserved. Do not reproduce without permission.




