Elon Musk's company, Neuralink, has approached one of the biggest neurosurgery centers in the US as a potential partner for clinical trials, according to Reuters.
(Photo : Nasa/Getty Images)
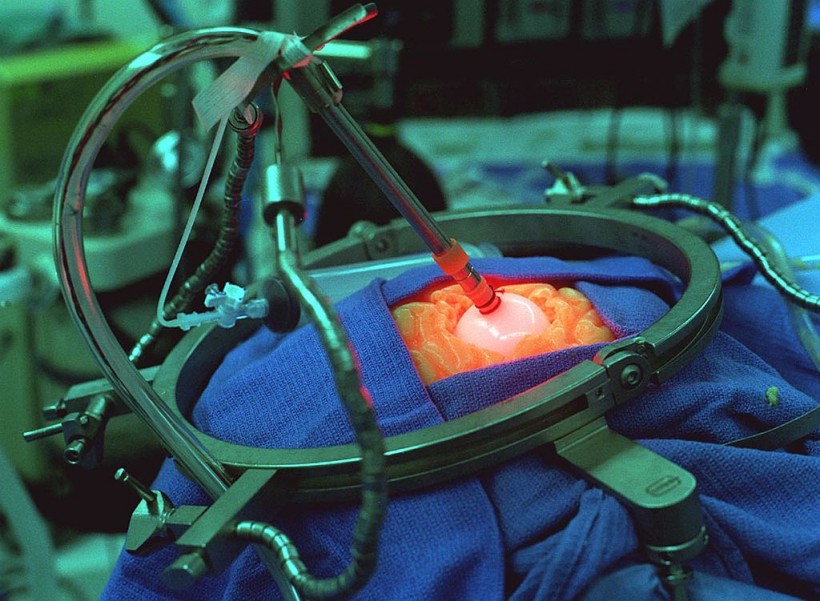
A Simulation Of Surgical Implantation Of The Light Emitting Diodes Probe At The Children's Hospital Of Wisconsin, Medical College Of Wisconsin, Milwaukee. The Led Probe Is Approximately 9 Inches Long And Is About One-Half Inch In Diameter.
The clinical trials are in preparation for Neuralink to test its devices on humans once regulators allow it. Musk's brain company has been developing brain implants since 2016 and hopes that they will develop a cure for intractable conditions, such as paralysis and blindness.
However, in 2022, the US Food and Drug Administration (FDA) rejected its application to progress to human trials due to safety concerns. The company has been working to address the agency's concerns.
On the Lookout for Centers
Neuralink is in talks with Barrow Neurological Institute, an Arizona-based neurological disease treatment and research organization, to help them execute human trials. However, it is unclear whether their talks will really turn into a solid partnership. That's why Neuralink is also talking to other centers.
In 2022, the US Department of Agriculture's Inspector General started looking into the potential animal-welfare violations at Neuralink. Former and current employees of the company detailed their concerns regarding the company's rushed experiments that led to needless suffering and deaths.
According to the US Department of Transportation, it is investigating potential mishandling of hazardous pathogens during Neuralink's partnership with University of California, Davis between 2018 and 2020.
Also Read: Top 5 Fears About Elon Musk's Neuralink: Mind Control, Hacking, Malfunction, Etc.
What Musk Says
The plan for clinical trials is part of Musk's vision for the development of Neuralink's brain chip. He plans to make the company's brain implants become as ubiquitous as Lasik eye surgery.
The company's brain implant is a Brain Computer Interface (BCI) device. It uses electrodes that penetrate the brain or sit on its surface to provide direct communication to computers. So far, no company has received US approval to bring a BCI implant to the market.
On the other hand, Barrow's implants are different from Neuralink's. Barrow uses deep brain stimulation devices that are approved by the FDA. Their devices help reduce Parkinson's tremors and have been implanted in over 170,000 patients.
The Neuralink device has a wide range of potential applications, including medical applications, such as treating neurological disorders, and non-medical applications, such as improving cognitive function or providing a direct interface between the brain and technology.
While the Neuralink device has the potential to revolutionize the way humans interact with technology, there are also concerns about the safety and ethical implications of brain implants. These concerns include the risk of infection, the potential for abuse, and the privacy implications of having direct access to a person's thoughts and emotions.
Related Article: Elon Musk Defends Neuralink Against Neuroscientist's Concerns of Chips Overheating










