Health Canada warned the public about the latest eye drops labeling errors. Since the product labeling issues are quite serious, the health department prompted a massive recall.
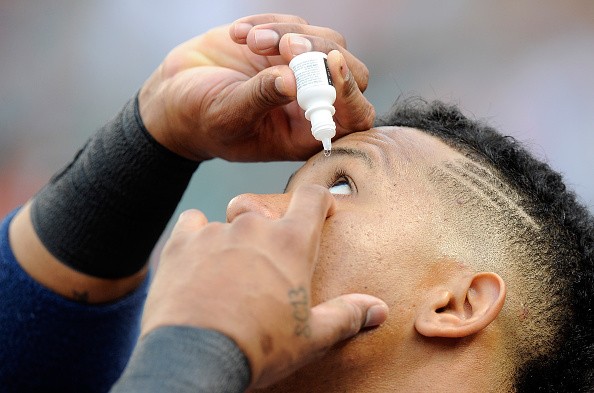
Teva Canada Ltd., one of the largest generic pharmaceutical companies in Canada, confirmed that it recollected its Compliments Advanced Relief Eye Drops and Pharmasave Advanced Relief Eye Drops because they were incorrectly packed.
This is a major concern since health products should be labeled properly so that people will know if they can use them without any health risks.
Health Canada Warns About Eye Drops Labelling Errors
According to Global News' latest report, the affected eye drop bottles are missing essential ingredient information, such as undeclared Naphazoline HCI or glycerine amount.
Also Read : Moderna COVID-19 Vaccine for 6-Month-Old Babies? FDA Authorization Achieved After Successful Trials
"Some bottles may contain ingredients that are not listed on the label and that may pose health risks to individuals who are allergic to the ingredients," said the Government of Canada via its official report.
Teva Canada's eye drops are quite popular since they are usually used for minor eye dryness, irritation, and burning.
This means that there's a high chance that some individuals have already used the incorrectly labeled eye drops were already used by some consumers.
For those who purchased the Canadian pharmaceutical company's eye drop products, the affected bottles are labeled with lot number RL21D01 or AR21C0.
What Should Canadians Do?
The Canadian Government stated that the recalled eye drops could trigger the allergies of consumers since they have naphazoline HCI or glycerine.
Symptoms may include itching and a rash. There are also some cases when people's faces, tongues, and throats are swelling.
If ever these signs are experienced by you or your family members, then here are the steps you need to follow:
- Reach out to Teva Canada Ltd. by calling their customer service (1-800-268-4129).
- As much as possible, report any signs of product-related side effects to Health Canada.
- Before using Teva Canada Ltd.'s eye drop products, consult your health professional first.
Meanwhile, a new COVID-19 study claims that seven out of ten individuals in England are infected.
On the other hand, experts discovered a new superbug that can transfer from pig to human.
For more updates about the latest eye drop labeling issue in Canada and other health topics, always keep your tabs open here at TechTimes.
Related Article : New Anti-Aging Vision Eye Drops Receive FDA Approval! Is This the Best Replacement for Eyeglasses and Contact Lenses?
This article is owned by TechTimes
Written by: Griffin Davis
ⓒ 2026 TECHTIMES.com All rights reserved. Do not reproduce without permission.




