Oxford scientists are raising hope that a breakthrough COVID-19 vaccine could be finally coming as they claim they are 80% confident that a jab could be ready two months from now. This comes after Moderna, an American Pharmaceutical company also announced that the vaccine they are working on is showing signs of immunity from the virus.
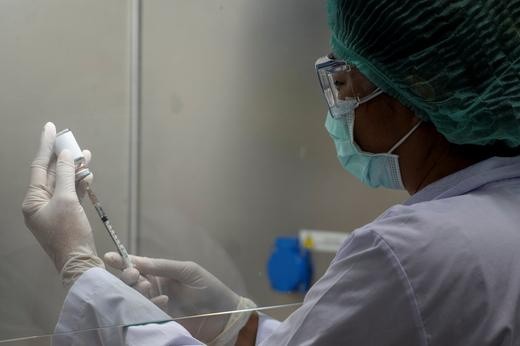
The subjects to the UK studies have been given Oxford Vaccines, which developed them the T-white blood cell, anti-bodies that help their bodies fight the COVID-19 virus if they get infected. Oxford's phase 3 trial has several 8,000 subjects across the UK, and 6,000 in South Africa and Brazil for these areas are more prone to the infection of coronavirus. The Oxford vaccine is currently being manufactured by AstraZeneca, based in Cambridge, England.
The Oxford Vaccine
The potential vaccine, which is named AZD1222, is already in Phase III human trials that assess whether it can protect humans against COVID-19. However, the discoveries have yet to report the whole data of Phase I results to show whether it is safe or induces an immune response. The developers of said vaccine were encouraged by the immune response they had seen in trials and now expecting to publish Phase 1 data by the end of July.
According to the World Health Organization's Chief Scientist, AstraZeneca's experimental vaccine is probably the world's leading candidate and most advanced in terms of development. AstraZeneca has signed agreements with Governments around the world to supply the vaccine once they clear it for widely use. The data of the vaccine are expected to be published by The Lancet medical journal.
The Vaccine's Data will be Published Next Week
"We expect this paper, which is undergoing final editing and preparation, to be published on Monday, July 20, for immediate release," a spokeswoman for the Lancet medical journal said.
Developers of the AZD1222 vaccine said that Phase I data will be published by the end of July. According to statistical reports, over 100 vaccines were being developed and tested worldwide to prevent the COVID-19 pandemic, which has killed hundreds of thousands and wrecked the global economy.
Moderna Vaccine
Moderna vaccine is also another promising vaccine being developed by US firm Gilead. However, after taking the trial vaccines there were more than half reported mild or moderate reactions such as fatigue, chills, headache, muscle, or pain at the injection site. But According to the scientists, those side effects were a 'small price to pay' for protection against Covid-19.
Dr. Anthony Fauci, the US government's top infectious disease expert, mentioned: 'No matter how you slice this, this is good news.'
The first US company, Moderna, was the one who started human testing of a vaccine for the novel coronavirus on March 16., just 66 days after the genetic sequence of the pathogen was released by China.
ⓒ 2026 TECHTIMES.com All rights reserved. Do not reproduce without permission.




