The "Adenovirus" ingredient found in COVID-19 vaccines from Johnson &Johnson (J&J) and AstraZeneca has been one of the top talks of the town as it causes alarm and a dangerous side effect. The use of the virus is to help the delivery and effectiveness of a vaccine as it enters the human body, making people immune to the disease to fight against it.
In the recent developments of the coronavirus pandemic, vaccines from Pfizer and BioNTech, and Moderna have been released for Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA). It has also been supported by the Center for Disease Control and Prevention (CDC), but new players like Oxford's AstraZeneca and J&J are slowly making their way.
The difference between these vaccines is their ingredients, and these components are integral in the development and approval of the vaccines in the country, as approved and studied by professionals. In reality, any vaccine would have unique effects on different people, as it would depend on a person's physique and health at the time they took it.
Read Also : Elon Musk COVID-19 Vaccine: Prefers J&J, BioNtech, Moderna, Suggests EpiPen for Allergic Reaction
J&J, AstraZeneca Uses Adenovirus
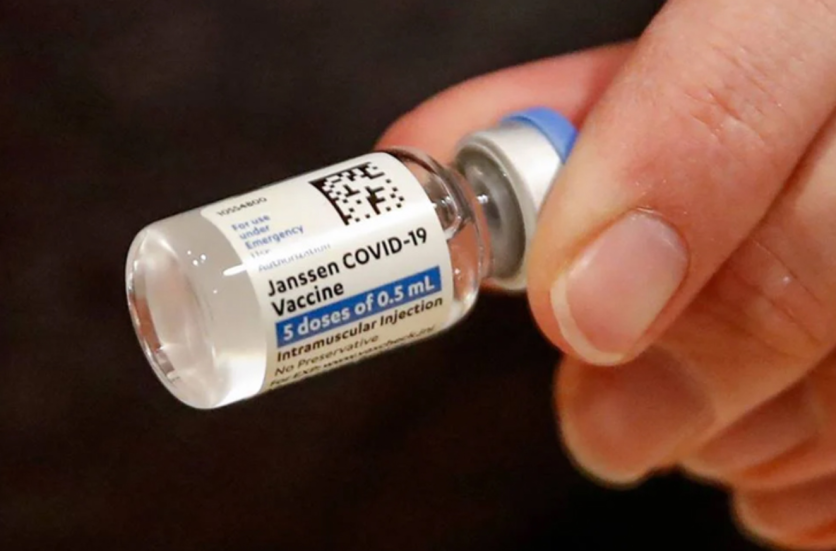
According to Ars Technica, both Johnson & Johnson and AstraZeneca are using the "Adenovirus" ingredient on their COVID-19 vaccines, which are known to be traditional components of the immunization shots. J&J uses the strain from the 1961 discovery known as "Ad26" found on children's anal swabs, while Astra bases it on a strain found on chimpanzees called "ChAdOx1."
Experts have linked both vaccines to bring isolated cases of unusual side effects, which have been linked to blood clotting for certain individuals in the country, hence FDA's decision to stop its administration. Currently, scientists and health experts are studying the vaccine and its effects, especially as both were not approved as EUA unlike Pfizer and Moderna's.
What is the 'Adenovirus'

According to the CDC, adenoviruses are commonly found strains of viruses that cause illnesses like sore throats, pneumonia, pink eye, bronchitis, and cold-like symptoms. The virus is commonly found in young ones, along with people that have weak immune systems to fight against the infectious component.
In a recent study by Nina H. Schultz and others, entitled "Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination," it was found that vaccines with adenovirus ingredient cause unique cases of reactions against the vaccine.
The FDA and CDC are trying to determine and look into the matter more, as there were no claims made by the country's health experts regarding its dangers or threats against human health. What is done, for now, is to stop its administration among the general public to avoid any serious implications, that have been linked to blood clots.
This article is owned by Tech Times
Written by Isaiah Alonzo
ⓒ 2026 TECHTIMES.com All rights reserved. Do not reproduce without permission.




