COVID-19 Vaccine for children is now the focus of the US Food and Drug Administration, as it has requested popular vaccine makers to develop one for the youngsters. This vaccine would be intended for children ages five to eleven, lower than the previous eligibility rating for the regular vaccines. However, it would be different from the earlier releases.
Initially, this topic was a hot case among the FDA and all vaccine creators in the country. Pfizer's studies have seen its vaccine effects for children ages 12 and above, which are all now part of the eligible ones. However, for those 5 to 11 years old, data and other studies made by Pfizer would have their first release in September.
COVID-19 Vaccine for Children
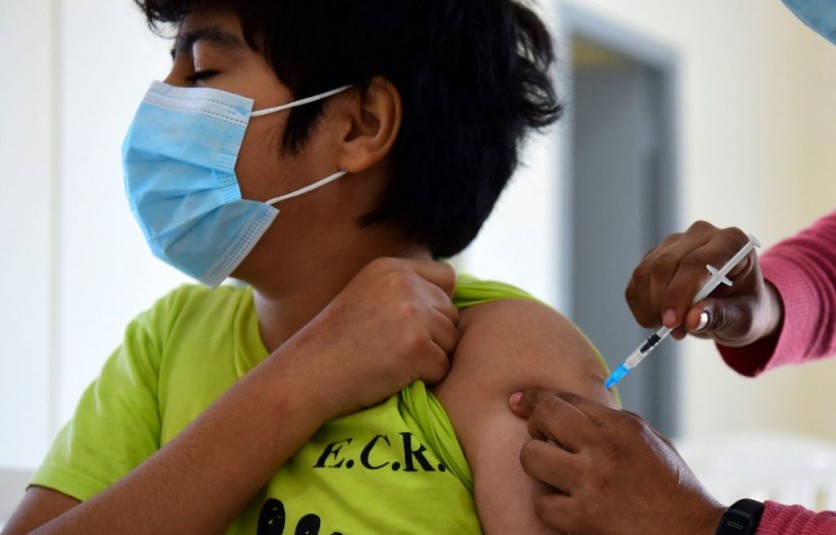
According to New York Times, the FDA has asked the help of the leading vaccine makers to formulate a new shot for children, so that it may help in protecting those younger than 12. The intended age target of this request is for children 5 to 11 years old. The request is asking for a new shot, and not a study if the existing shot can be used.
Children which fall under 11 years old and below are considered to have a developing immune system, and getting the full blast of the vaccine intended for adults may be of harm. That being said, the FDA is now looking into Pfizer & BioNTech and Moderna for these shots.
The focus would be on mRNA ingredients, which are considered to be the ones with fewer side effects.
FDA wants Vaccine Makers to Formulate Shots
The vaccine for children would be the priority and not the booster shots which Pfizer was initially pushing for, especially during this time where the Delta Variant is rampant in spreading. Children would soon be part of the immunity campaign, as said by the FDA.
The FDA has no mentions of the COVID-19 vaccine for children in its recent press announcements, but it would soon be addressed by the health authorities in time.
Furthermore, the growing panic amidst COVID-19's infection rate for now, and the return of CDC's mask mandate in public has people on mixed terms with the government.
Is It Safe?
There are no vaccines for children available as of the moment, particularly for those under 12 years old. However, it would go to a lot of testing phases and studies before it even makes it to the FDA. And with the FDA, it would still go under testing before a EUA is issued for the company.
That being said, the COVID-19 Vaccine for Children would be safe, if deemed worthy and accredited by the health authorities and experts from the Food and Drug Administration.
Related Article : COVID-19 Resurgence Prompts CDC Reconsiders Face Mask Mandate
This article is owned by Tech Times
Written by Isaiah Richard
ⓒ 2026 TECHTIMES.com All rights reserved. Do not reproduce without permission.




