Pfizer's Vaccine for COVID-19 was reported to have been approved for Emergency Use Authorization by the US F.D.A. for adolescents in the 12 to 15-year-old age range and would start on Wednesday, May 12. The EUA was approved more than a week ago, and it is a massive development for the country's vaccination drive to immunize against the coronavirus.
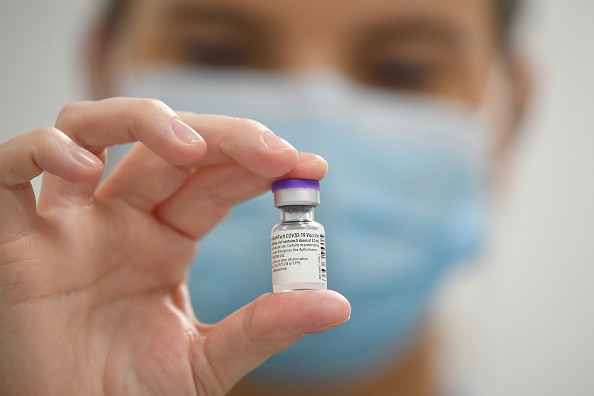
From the legal age of 18, down to sweet 16, and now coming for 12 to 15-year-olds, Pfizer and BioNTech's mRNA-based COVID-19 vaccine is slowly being distributed alongside the country's return to normal with its immunization. Initially, the vaccine for adolescents was approved by the FDA at the beginning of May, and it was only for the shot made by Pfizer.
Moderna's COVID-19 vaccine which uses the same mRNA background is still undergoing tests and it promised that it would reveal it by summer. Nonetheless, the GenXZeneca craze in Canada would not likely be the case for adolescents in America, as the need for parental consent and patronage while receiving the shot would be the case here.
Read Also : No Vaccine No Entry | Over 100 US Colleges & Universities Require Students to Get Vaccinated
Pfizer Vaccine Eligibility for 12 to 15-Year-Olds
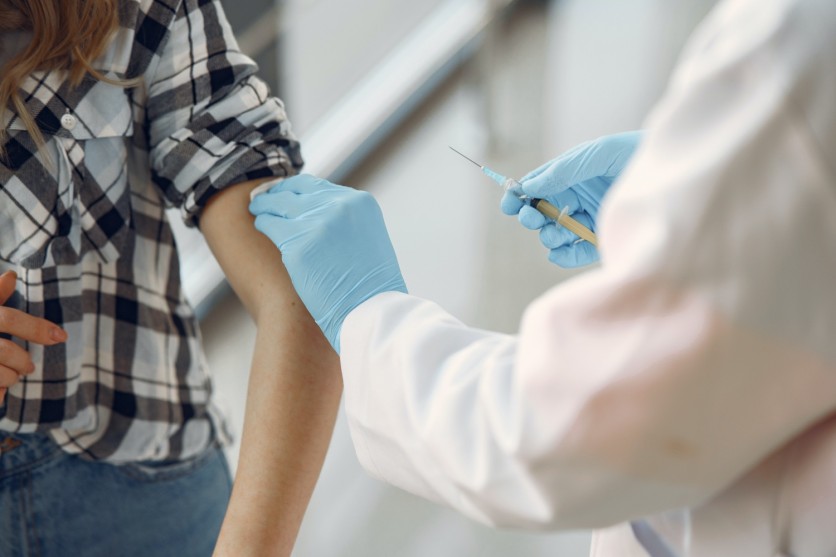
Slowly but surely, that is how the COVID-19 vaccine is passing from the legal age of eligibility, down to adolescents who are in the younger age zone from 12 to 15-year-olds. While younger immune systems are stronger, they are still subject to getting infected and suffering dire consequences if studies were not made before administration.
Good thing is that the Food and Drug Administration, along with the CDC, has been studying the effects it would have for the younger age group, apart from Pfizer's studies, and found it to be ready for use. According to the FDA's report, shots for adolescents may be available by Wednesday, May 12, to start the program and immunize children.
According to Boston.com, adolescents have been found to develop more robust antibodies against COVID-19 with the vaccine.
Schools to Reopen Soon?
This is part of the ongoing effort of the country to rehabilitate itself after the modern-day pandemic has struck and pushed it down its knees which almost shut down everything. The vaccine development and availability in the country have been coming in at a steady rate, hence its administration to regular citizens, and now even for adolescents.
Children to receive the shots would most likely be allowed to return for a face-to-face setup in school and continue learning for a new school year without the worry of catching the coronavirus outside. This would be the country's fight and return from the devastating attack and effects of the pandemic.
Related Article: Pfizer COVID-19 Vaccine: 'Extremely Effective' Against UK and South Africa Variants' Harmful Effects, Not a Booster Like Moderna
This article is owned by Tech Times
Written by Isaiah Richard
ⓒ 2026 TECHTIMES.com All rights reserved. Do not reproduce without permission.




